Create the Participant Registry
Learning Objectives
After completing this unit, you’ll be able to:
- Describe the Participant Management data model.
- Explain how to set up the search configuration to make clinical trials searchable.
- Describe how Experience Cloud allows users to securely search for trials.
Move from Awareness to Action
In the world of clinical trials, a participant registry acts as a central hub, connecting potential participants with suitable research studies. This makes it easy for individuals to find and join trials according to their health conditions and interests.
Learn how Participant Management for Life Sciences Cloud puts patients in the driver's seat of their clinical trial journey by following the experience of Milly Lee, a potential trial participant.
Milly’s path to participation starts by conversing with her doctor, who tells her about a new influenza trial sponsored by Cumulus Pharma. Her doctor learned about this opportunity through a healthcare provider engagement app developed by Cumulus Pharma to connect with healthcare providers. Inspired to contribute to such promising research, Milly visits Cumulus Pharma’s recruitment portal, powered by Salesforce Experience Cloud.
The portal makes it easy to find relevant trials. Milly simply searches for “influenza” and quickly discovers a list of options.
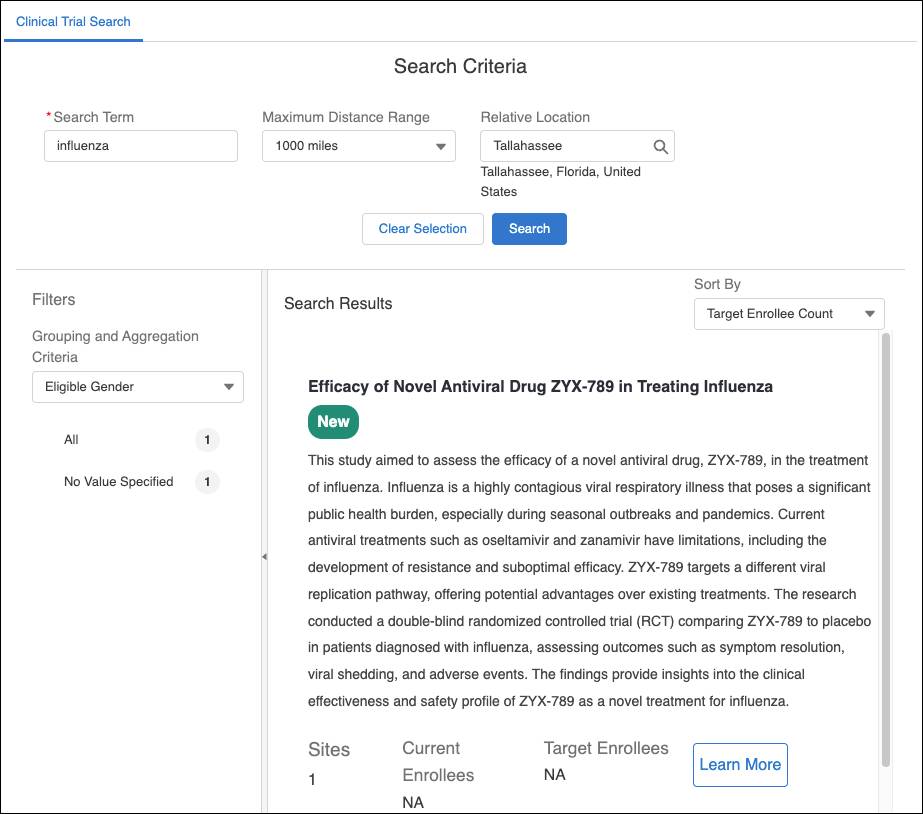
A promising influenza trial catches her eye, and she clicks Learn More to review details about the study.
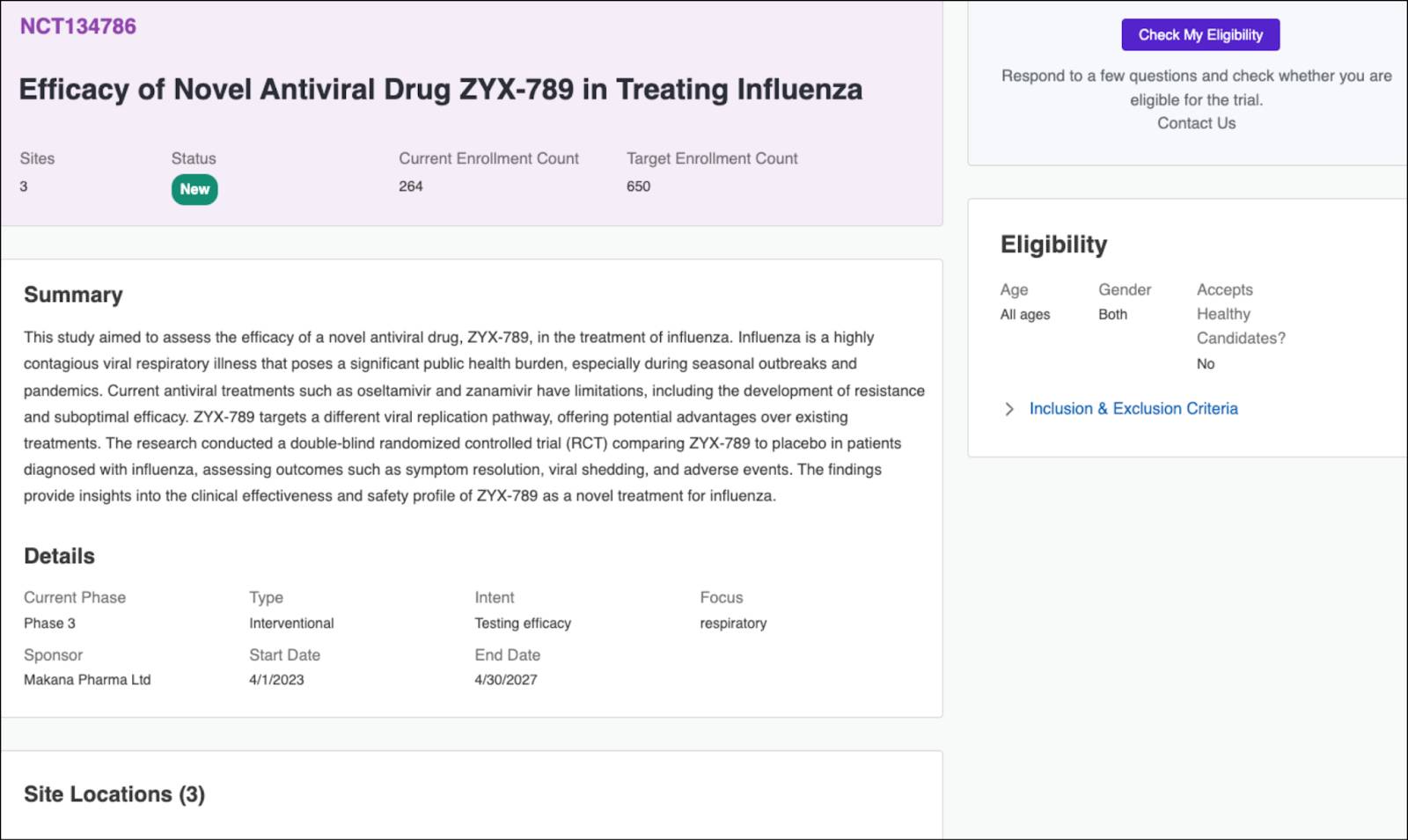
This trial summary page provides a concise overview, including the study summary, a list of participating sites, target enrollment numbers, and eligibility and participation requirements.
While Milly’s experience seems effortless, it’s the result of careful planning and execution by the Cumulus Pharma team. To prepare for participant recruitment and enrollment across multiple sites, different teams at Cumulus Pharma have been busy.
-
Recruitment: The Recruitment Strategy team crafts a detailed plan to identify and engage potential participants.
-
Technology: Justus, the Salesforce admin for Cumulus Pharma, and his team configure data streams, map data objects, and define target segments to align with the recruitment strategy. They also set up the central trial recruitment portal and site-specific Patient Registry and streamlined enrollment workflows.
-
Marketing: The marketing team launches patient awareness campaigns using Salesforce Campaigns to inform the public about ongoing trials and drive traffic to the recruitment portal.
A coordinated effort ensures that the right information reaches the right people at the right time, creating a personalized experience for potential participants. Follow along as Cumulus sets up the necessary components to create a powerful tool for accelerating clinical trial enrollment.
Set Up the Foundation
Let’s explore the Participant Management data model and how it’s tailored for research studies like Cumulus Pharma’s influenza trial. See how it integrates with care programs to offer additional capabilities such as centralized data management and enhanced collaboration.
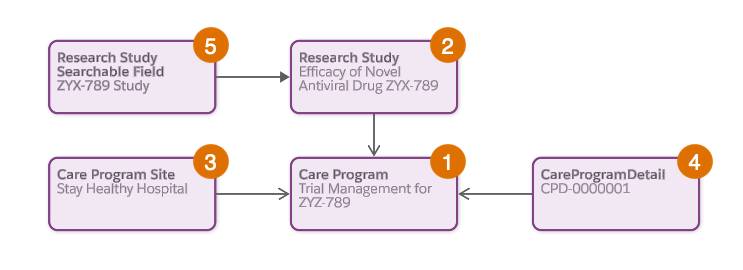
The table describes the objects and their relationships.
Object |
Description |
Scenario |
|---|---|---|
Care Program (1) |
Represents a set of activities offered to patients. Define eligibility rules and criteria to qualify participants for the program. |
Cumulus Pharma creates a Care Program record for the research study, categorizing it as Trial Management to enable clinical trial-specific fields and relationships. |
Research Study (2) |
Stores detailed information about the research study, including design, execution, and oversight. |
Cumulus Pharma creates a Research Study record to assess the efficacy of the Novel Antiviral Drug ZYX-789 in treating influenza, associating the record with the Care Program. |
Care Program Sites (3) |
Captures information about the locations where the care program is offered, including name, related care program, and healthcare facility. |
Cumulus Pharma creates multiple care program sites for this study, associating them with the influenza study care program. |
Care Program Detail (4) |
Depending on the detail type, can perform two functions:
|
Cumulus Pharma creates two Care Program Detail records:
|
Research Study Searchable Field (5) |
Represents a common dataset with fields and values from multiple objects, and is used as the basis for searches related to research studies. |
When Cumulus Pharma executes a Data Processing Engine definition for the clinical trial, this record stores data from multiple objects in a single searchable format. |
As you explore the next stages of participant management, you’ll encounter additional objects essential to the model. Understanding these core objects helps you grasp how Participant Management guides participants through the clinical trial process.
Enable Discovery
To ensure users can easily find relevant clinical trials among the vast amount of Salesforce data, Justus turns to a powerful tool: Data Processing Engine.
Think of Data Processing Engine as a behind-the-scenes wizard. It gathers data from different objects and transforms it into a standardized format using the Research Study Searchable Field object. This object is the foundation for criteria-based search, allowing users to filter and refine their search based on characteristics like study phase, therapeutic area, patient demographics, and more.
To set up Data Processing Engine and enable criteria-based search for clinical trials, follow these steps.
-
Enable permissions: Ensure that the necessary permissions are in place, including the Clinical Trial Manager and Data Pipelines Base User permission sets.
-
Activate the features: Enable the Data Pipelines and Criteria-Based Search & Filter features in Salesforce Setup.
-
Customize data processing engine: Clone and activate the "Populate Research Study Searchable Field" Data Processing Engine to tailor it to your organization’s specific data model and search requirements.
-
Configure the searchable object: Create a searchable object configuration, and link it to the customized Data Processing Engine to synchronize data between the source objects and the ResearchStudySearchableFields record.
-
Set search criteria: Establish the search criteria configuration, specifying which fields are searchable, how results are displayed, and any grouping or aggregation options.
To learn more about setting up Data Processing Engine to support clinical trials, review this help topic: Criteria-Based Search and Filter.
After these configurations are in place, Data Processing Engine works in the background to keep the Research Study Searchable Field record up to date. Whenever new clinical trials are created or updated, the engine automatically extracts and transforms the relevant data, ensuring search results are always accurate and comprehensive.
For Cumulus Pharma’s influenza trial, this means potential participants can quickly and easily find the trial using specific criteria. They can filter by study phase (Phase III), therapeutic area (infectious diseases), patient demographics (age, gender, location), and other relevant details. This targeted search capability saves valuable time and allows potential candidates to make informed decisions based on the most relevant clinical trial information.
Build the Gateway
Use Experience Cloud so that potential trial participants can easily find and enroll in suitable clinical trials. By setting up a dedicated site with guest user access, candidates can securely search for trials, complete screening questionnaires, and initiate the enrollment process.
Creating this user-friendly portal involves several key steps.
-
Enable digital experiences: Unlocks the full potential of Salesforce Experience Cloud within your organization.
-
Build a community site: Provides a secure space for guest users to interact with your content and applications.
-
Configure guest user permissions: Allows guest users to access relevant trial data through interactive components like Flexcards and Data Raptors.
Justus follows these steps to set up a portal for Cumulus Pharma so participants like Milly can easily search for clinical trials.
In addition to the criteria-based search and filter widget, Life Sciences Cloud offers prebuilt Flexcards to speed up portal development. You can easily customize the reusable components to display trial information, capture participant data, and guide users through the enrollment process. Here are just a few of the components.
Flexcard |
Description |
|---|---|
TrialManagementResearchStudyHighlights |
Displays summary information about a research study. |
TrialManagementResearchStudyDetails |
Shows more detailed information about a research study. |
TrialManagementResearchStudySites |
Displays details about a research study site. |
TrialManagementResearchStudyLaunchEligibilityCheck |
Launches a workflow that checks eligibility for a clinical trial. |
TrialManagementTrialEligibilityCriteria |
Shows candidates the eligibility criteria for a trial. |
After setting up the portal, you can configure sharing rules to manage user access to data based on their role or status in the trial. These rules are crucial for ensuring data security and privacy throughout the trial's duration.
See a detailed walkthrough of the Experience Cloud site setup process.
In this unit, you learned how the Participant Management data model, criteria-based search, and Experience Cloud work together to create a comprehensive recruitment experience for potential trial participants.
In the next unit, learn how to use orchestration and workflow tools to create an equally smooth screening and application process.
Resources
- Salesforce Help: Participant Management Data Model and Permissions
- Salesforce Help: Add Data for Participant Recruitment and Enrollment
- Salesforce Help: Discover Clinical Trials Easily with Criteria-Based Search and Filter
- Salesforce Help: Set Up Experience Cloud Site for Participant Management
- Salesforce Help: Create Authenticated Users Sharing Rules in Participant Management