Discover Regulatory and Commercial Realities
Learning Objectives
After completing this unit, you’ll be able to:
- Explain how medical devices are classified by risk and what that means for regulatory review.
- Compare how major regulatory bodies approach device approval.
- Describe how regulatory timelines and local requirements shape global launch strategies.
- Identify how healthcare systems, reimbursement structures, and pricing models affect market access.
Same Goal, Different Paths
MedTech improves lives, but because these products directly affect health, regulation is critical. Around the world, governments set strict rules to ensure medical devices are safe and effective before they reach patients. While details differ by country, most regulators use a risk-based classification system. Typically, devices are classified into classes based on potential risk to patients.
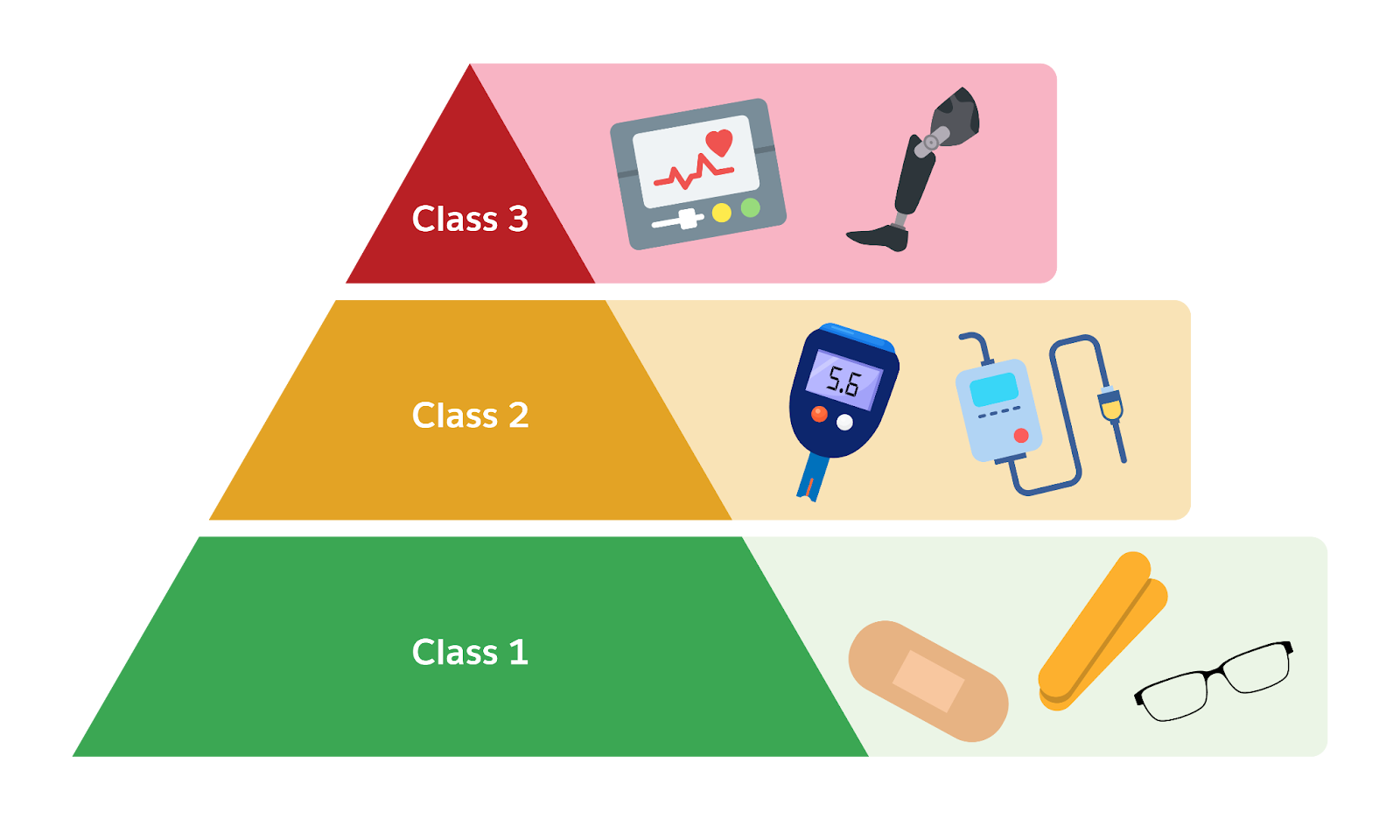
-
Class I (Low Risk): These devices pose minimal potential harm. Examples include bandages, tongue depressors, and reading glasses. Most Class I devices don’t require lengthy approval processes. Manufacturers might just register them and follow basic quality standards.
-
Class II (Moderate Risk): These devices are moderately complex or invasive. Examples include infusion pumps, motorized wheelchairs, and blood glucose monitors. Regulators usually require evidence that these devices are safe and work as intended, but often through a streamlined process. For instance, in the US, many Class II devices go through the FDA’s 510(k) premarket notification pathway, where a company shows the new device is substantially equivalent to an already approved device.
-
Class III (High Risk): These support or sustain life, or are implanted in the body, and carry the highest risk. Examples include pacemakers, artificial hips, and implantable defibrillators. Class III devices typically need rigorous premarket approval, which involves detailed review of clinical trial data and manufacturing processes. Regulators leave no stone unturned here because patient safety is on the line.
Different regions have their own notation, but the principle is the same: Higher risk equals stricter oversight.
It’s not just about getting approval. MedTech companies also face regulations on manufacturing quality, post-market surveillance, and more. In many countries, regulators continue to monitor devices once they’re on the market, ensuring companies report malfunctions or recalls promptly. All this adds complexity and cost for MedTech firms, but it’s essential for protecting patients.
All major markets share the same goal: safe, effective devices. But the approval pathways vary widely. Understanding these differences enables MedTech companies to build a global strategy.
This table compares a few major regulatory bodies.
Regulatory timelines strongly influence launch strategy. For years, many companies launched first in Europe as CE Marking was once faster and less costly. But the 2021 EU Medical Device Regulation (MDR) introduced stricter requirements and longer reviews.
Today, the US is often the first market for product launches. This is because the FDA’s structured pathways and expedited programs are more predictable for novel products. But each launch plan depends on the product and business goals.
Consider a new surgical device. If US review is faster and the market is large, the FDA route may come first. If clinical studies are already underway in Europe, CE Marking could be the quicker path. Some firms now submit in multiple regions at once to stay flexible.
The right regulatory sequence often sets the tone for your global commercial rollout.
Healthcare Systems
In the EU, each country negotiates its own pricing and coverage, even after CE Marking. Health systems expect strong cost-effectiveness evidence. Centralized evaluations, such as Germany’s Gemeinsamer Bundesausschuss (G-BA) and UK’s National Institute for Health and Care Excellence (NICE), can delay uptake, but once approved, adoption is often systemwide.
In the US, reimbursement is fragmented across public and private payers. Medicare coverage is critical for seniors. Private insurers follow varying timelines and policies. Winning coverage codes can be complex, but successful US reimbursement can unlock huge revenue potential.
In China, price and access depend on local procurement systems. Regional tenders and decision-making at the hospital level add complexity. Even after approval, market entry often requires strategic partnerships.
Across all markets, real-world evidence and health economic studies are key to convincing payers and providers.
Access also depends on operations. Many markets require a local footprint.
- In the EU, non-EU companies must appoint an Authorized Representative.
- In China and Brazil, companies typically require a local agent or subsidiary.
- In Japan, market authorization holders (MAHs) are needed for regulatory interface.
These logistics affect cost, lead time, and how companies manage their commercial teams. Planning for localized compliance support is part of go-to-market readiness.
Launching Commercial Strategy with Market Realities
Every launch is a strategic choice. MedTech companies weigh regulatory timelines, healthcare infrastructure, patient demand, and business goals to decide where and when to introduce a product.
Key considerations include:
-
Time to approval: Where will regulatory review be fastest or most predictable?
-
Market potential: Where’s the greatest demand or unmet need?
-
Pricing environment: Where can the device achieve sustainable reimbursement?
-
Operational readiness: Where do we already have partners or teams in place?
A common strategy: Launch in the US to secure early revenue, then use clinical and commercial data to accelerate approvals elsewhere. Or for faster access, target a CE mark and use it for recognition in Middle Eastern or APAC markets.
Some companies test new devices through pilot programs with leading hospitals. Others build budget impact models to support pricing discussions with ministries or insurers.
The Commercial Model Is Evolving
Device makers aren’t just changing where they launch—they’re rethinking how they sell.
Capital equipment, once sold as a one-time purchase, is increasingly offered through “as-a-Service” models. Hospitals can now lease surgical robots, or pay per procedure, rather than face a multimillion-dollar upfront cost.
This shift helps providers manage budgets and gives manufacturers recurring revenue. It also aligns incentives: If the device doesn’t perform, the buyer isn’t locked in. In some cases, contracts are even outcome-based, as the device must deliver results to trigger payment.
These models are especially valuable in regions with budget constraints or public health systems. And they support subscription billing, usage-based pricing, or service contract tracking.
Context Is Everything
MedTech is global, but success is local. Launching the same device in two different countries can require entirely different playbooks.
Understanding how regulatory frameworks work—and how they interact with healthcare systems and reimbursement—helps MedTech companies time their launches, build pricing strategies, and choose the right commercial model.
As a MedTech organization, your solutions must reflect that diversity. Whether you're supporting field service tracking, global sales enablement, or patient engagement tools, region-specific workflows and compliance are key.
In the next unit, explore how these market realities intersect with the technologies reshaping MedTech, from connected care to AI, robotics, and beyond. The future is coming fast. Let’s take a look.