Meet Participant Management
Learning Objectives
After completing this unit, you’ll be able to:
- Define participant management in the context of clinical trials.
- Describe the main challenges of participant management.
- Explain the key features and benefits of Participant Management for Life Sciences Cloud.
- Describe the main jobs supported by Participant Management for Life Sciences Cloud.
Before You Start
Before you start this module, consider completing this recommended content..
Efficiency in Clinical Trials
Ever wondered how life-changing medicines and treatments become available to the general public? What has to happen, for instance, to place a new antiviral treatment on the shelves of your local pharmacy? Often, a clinical trial has taken place to make sure the treatment is safe and effective.
In addition to a good research design and proper funding, the success of any clinical trial depends on the efficient recruitment and enrollment of participants. This process involves a series of steps—from initial outreach to obtaining informed consent—which are essential for assembling a representative participant base. However, clinical trial teams often face significant hurdles. Delays in enrollment, escalating costs, operational inefficiencies, and a suboptimal participant experience all form setbacks in developing life-saving treatments.

At the core of these trials are two critical entities: sponsors and investigative sites. Sponsors, typically pharmaceutical companies or medical research organizations, are responsible for the trial’s overall management, financing, and regulatory compliance. Investigative sites are where the actual research takes place, involving direct interaction with participants.
Challenges like meeting enrollment targets and maintaining efficient operations can create bottlenecks that affect the entire trial process. Delays and bottlenecks impose financial burdens on organizations and can prevent patients from accessing life-changing interventions. If you've ever had a loved one with a serious illness, there's nothing more important than getting them the best treatment ASAP. And this is why clinical trials and participant management are critical.
Meet Participant Management
Participant Management for Life Sciences Cloud equips life sciences organizations with a data-driven solution for delivering personalized experiences for everyone involved in clinical trials.
-
Streamline recruitment: Quickly match candidates to eligible trials, simplify informed consent, and efficiently screen qualified participants to reduce time-to-enrollment.
-
Drive efficiencies: Leverage data on study protocols, site performance, and real-world patient information to refine protocol creation and accelerate site startup. Use AI tools for inclusion and exclusion criteria, site recommendations, and precise candidate matching.
-
Automate complex workflows: Orchestrate end-to-end clinical processes like site selection, participant enrollment, and milestone tracking.
-
Personalize experiences: Tailor experiences for patients, sites, and sponsors through dedicated portals, apps, and dashboards.
Together, these capabilities minimize the time from initial contact to final enrollment, significantly improving trial efficiency, effectiveness, and overall success. That means better treatments available sooner.
Participant Management isn't just a standalone solution; it's a core component of the unified clinical platform within Life Sciences Cloud. This comprehensive platform enables smarter trial design and execution across the entire clinical trial lifecycle, including Site Management (where sponsors find and assess investigative sites) and flowing directly into the participant journey.
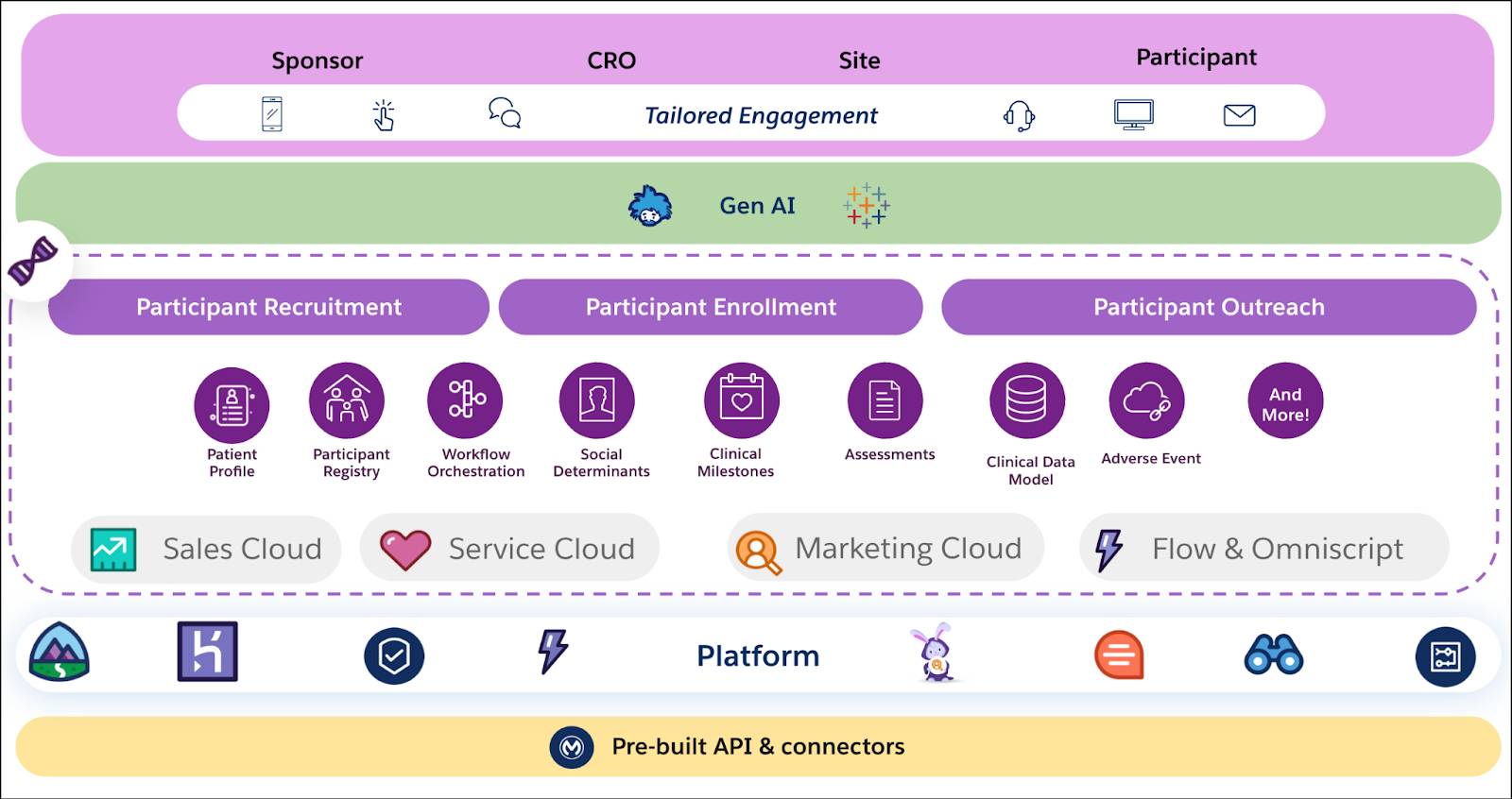
This integrated approach uses prebuilt applications, industry-specific data models, and flexible integrations to streamline every aspect of clinical trial operations. Within this framework, Participant Management directly addresses the critical challenges of recruitment and enrollment, ensuring you can identify and engage qualified participants throughout the trial journey.
Here are some key features of Participant Management.
Feature |
Description |
|---|---|
Industry-Aligned Data Model |
Store and manage data in a format that is fully compatible with other healthcare systems and adheres to FHIR R4 and USCDI standards. |
Criteria-Based Search and Filter |
Enable candidates to easily find trials that align with their specific criteria. |
Omnistudio Guided Processes |
Customize prebuilt Omniscripts to digitize and streamline prescreening and application processes for a seamless participant experience. |
Stage Orchestration |
Orchestrate the entire enrollment journey—from initial contact to randomization—with enhanced visibility and control. |
Consent Management Flows |
Automate the informed consent process with prebuilt flows, ensuring compliance and reducing the administrative burden. |
Invocable Actions for Randomization |
Automate the randomization process, ensuring unbiased assignment of participants to treatment groups. |
Follow the Clinical Trial Journey
Cumulus Pharma, a leader in pharmaceutical innovation, is poised to launch Phase 3 of its influenza antiviral drug trial. With plans to conduct the trial across multiple sites in the USA, the Cumulus Clinical Trial team outlines a comprehensive recruitment and enrollment strategy.
And who can deliver on this strategy? Justin Pardo, adept Salesforce administrator, and his trusty team—they’re perfect for the job.

It’s his mission to roll out a secure and efficient recruitment and enrollment workflow for prospective participants and investigative sites. Justus has to enable criteria-based search, establish a central recruitment portal, create screening assessments, and ensure regulatory compliance… no small ask!
Justus will harness the power of Participant Management tools to create a smooth journey for everyone involved. Future participants will be able to explore exciting new trials, check if they’re a good fit, and register their interest. Meanwhile, the dedicated recruiters at the study sites will use the solution to find and connect with potential participants. They’ll guide them through the informed consent process, set up those first screening appointments, and keep a close eye on how many people are joining the study.
In the next unit, join Justus as he sets up the essential components that enable trial candidates to search for eligible trials. You also learn more about the data model and how it underpins participant recruitment.