Get to Know Pharma Industry Basics
Learning Objectives
After completing this unit, you’ll be able to:
- Define the role of the pharmaceutical industry in healthcare and life sciences.
- Outline the lifecycle of drug development from research to market.
- Identify the main business objectives of pharmaceutical companies.
- Define key terms and regulations in the pharmaceutical sector.
The Pharmacist Is In
If you live in a big city and need medicine at 2 AM, you’re grateful for the 24-hour pharmacy a short drive away. But if you’re a small-town resident, access to a round-the-clock drugstore might not be an option. In this case, you plan ahead when possible, obtaining prescriptions in advance from your clinic. Otherwise, it can be a long wait until morning when the pharmacy opens.
Wherever you reside, it’s likely you’ll need medicine sometimes. So in this way, we rely on the pharmaceutical industry. Pharmaceutical manufacturers are part of the life-sciences sector, which focuses on researching and producing drugs and medical devices to improve health.
Pharmaceutical, or pharma, companies are one of five core healthcare sectors, alongside providers, payers, MedTech, and public health. Hospitals and doctors are examples of providers, and insurance companies are types of payers. MedTech covers medical device and technology makers, while public health entities are government agencies and services. In this module, you focus specifically on the pharma sector. For an overview of all five sectors, check out The Healthcare and Life Sciences Industry: Quick Look.
Let’s see pharma in action for Alvin, a dog walker. One day, his dogs chase a squirrel, and he gets tangled in the leashes. He falls and breaks his leg, and within the hour, he’s in the emergency room. When he gets a painkiller, Alvin enters the world of pharma.

The doctor who prescribes the painkiller, the pharmacy that fills it, and the manufacturer that produces it are all part of the pharmaceutical ecosystem.
The Drug-Development Lifecycle
Pharmacies have existed for centuries, yet the core goal of pharmaceuticals remains the same: to discover, develop, produce, and market drugs that prevent, treat, or cure disease. Today, companies apply advanced science to create everything from vaccines to antibiotics to chronic condition medications.
From Alvin in the US receiving pain meds, to a child in Japan getting a vaccine, or a grandmother in France managing diabetes, pharma touches lives around the world.
But before any of those drugs reach patients, they go through a long, complex journey. This lifecycle is expensive, tightly regulated, and high risk. It typically takes 10–15 years and often billions of dollars to bring a single new drug to market. From thousands of candidates, only one might earn approval.
Key stages in the process include:
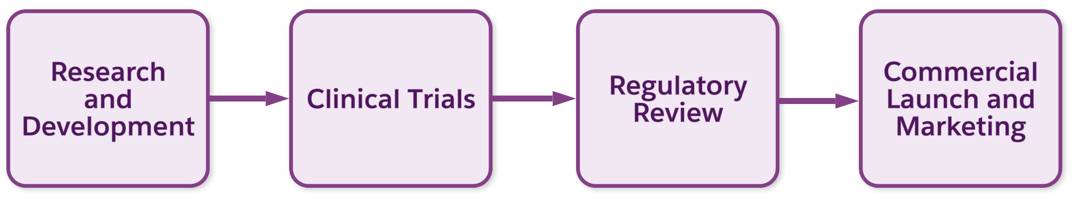
-
Research and development (R&D): Scientists search for and synthesize promising compounds, then test them in labs for safety. This is the creative heart of pharma, where innovation happens.
-
Clinical trials: Next comes testing new therapies on humans, typically in phases. Trials start with small safety groups and then expand to large populations to confirm effectiveness and monitor side effects.
-
Regulatory review: Government agencies like the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) evaluate all the evidence. Approval can take months or years and involves detailed back-and-forth with regulators.
-
Commercial launch and marketing: After drug approval, companies manufacture at scale, distribute globally, and market to healthcare providers. Sales reps educate doctors on proper usage, and marketing campaigns, where allowed, raise awareness.
Once a medicine is launched, payers decide on coverage, providers choose when to prescribe it, and patients must follow the instructions for use.
You’ll explore the stakeholders in the next unit. For now, learn what pharma companies aim to accomplish.
What Pharma Companies Care About
Across departments—R&D, regulatory, marketing, and sales—pharma companies have common business goals:
- Discover and develop novel, differentiated therapies for unmet needs.
- Meet quality and regulatory standards.
- Secure coverage from payers and access for patients.
- Achieve commercial success to fund future work.

Explore how these goals come into play across the drug lifecycle.
R&D
In the R&D stage, the goal is to discover new treatments, especially for conditions that currently lack effective therapies or have limited treatment options. The set of potential drugs in development is known as the pipeline, and a brand-new drug candidate is called a new molecular entity (NME). A strong pipeline with promising NMEs improves the odds that at least one will succeed. But companies aim for quality over quantity, as they don’t want to crowd the pipeline with low-potential projects.
Instead, they prioritize compounds that:
- Address clear gaps in treatment.
- Work better than current options or offer a novel mechanism of action.
- Have a realistic chance of regulatory approval.
Drug development is expensive and risky. It can take billions in investment across research, trials, and regulatory review, often with many failures along the way. Only a small percentage of ideas make it to market. That’s why pharma companies continually assess scientific viability, patient benefit, and commercial potential, cutting programs that fall short. It may sound harsh, but it ensures that the drugs moving forward are the ones most likely to be safe, effective, and meaningful. In life sciences, innovation is about impact not volume.
Clinical Trials and Regulatory Review
It can take many years and an enormous budget to get through clinical trials and regulatory approvals. To speed things up, companies use digital tools to identify and enroll eligible patients quickly, coordinate trials across global sites, and capture data accurately and efficiently. The goal is faster, smarter trials that still meet the highest standards, so that promising therapies reach patients sooner.
When it comes to regulatory review, compliance is critical. Companies must submit comprehensive data packages and follow the specific rules of each region. Any delay in approval means patients are left waiting, and the company isn’t generating revenue. It’s a delicate balance: Avoid unnecessary delays, but never cut corners on safety.
Commercial Launch and Marketing
Once a drug is approved, the focus shifts to ensuring that patients can access and benefit from it. Pharma companies work on several fronts:
-
Engaging healthcare providers (HCPs): Pharma companies educate doctors, pharmacists, and nurses on the drug’s benefits, usage, and safety. A physician is more likely to prescribe a treatment when they understand how and when to use it effectively.
-
Working with payers: They negotiate with insurers and health systems to include the drug on formularies. A formulary is a list of covered medications. For example, Alvin’s painkiller must be listed on the formulary to be covered under his plan. Pharma companies have to demonstrate the drug’s clinical and economic value, especially in countries where governments regulate pricing or require cost-effectiveness studies.
-
Supporting patients: The journey doesn’t end with a filled prescription. Pharma companies often provide patient services programs to help them start and stick with treatment. Programs include nurse hotlines, injection training, co-pay assistance, free samples, and condition-specific education.
-
Managing supply chains: Pharmacies, clinics, and hospitals must be reliably stocked through coordinated distribution and logistics, another responsibility of the pharma company.
At the commercial stage, pharma’s objectives of patient benefit and business sustainability come together: A drug that genuinely helps patients, and is priced reasonably, will gain wider use, which in turn drives the company’s revenue. In the end, a pharmaceutical company measures its success not just in dollars earned, but in the health outcomes achieved.
The Culmination of a Successful Medicine
Getting back to Alvin—when he received the prescription painkiller for his broken leg, the pharmaceutical sector was already deeply involved. A pharma company had researched and developed the drug, proven its safety and efficacy in trials, and secured regulatory approval. The drug was manufactured at scale and marketed it to the doctor who treated Alvin. Behind the scenes, the pharma company likely worked with Alvin’s insurer to verify that the drug was covered under his plan.
It’s a long, carefully orchestrated journey from scientific discovery to bedside relief. And while it’s complex, every step is aimed at the same outcome: getting the right treatment to the right patient, at the right time.
That’s not everything pharma does, but it’s a solid foundation. Next, look closer at what these companies actually produce, and meet their global customers and partners.