Deliver Clinical and Advanced Therapy Excellence
Learning Objectives
After completing this unit, you’ll be able to:
- List the current challenges facing clinical operations in life sciences.
- Discuss how Life Sciences Cloud supports teams in coordinating clinical research and therapy.
- Describe how Life Sciences Cloud supports site selection, site activation, and study management.
- Summarize how the platform improves participant recruitment and enrollment.
- Identify how Advanced Therapy Management supports orchestration and custody for complex treatments.
Deliver Modern Clinical and Therapy Execution
Today’s life sciences organizations are navigating an increasingly complex care and research landscape. From traditional clinical trials to personalized therapies like CAR T-cell treatment, teams must coordinate high-stakes activities across sites, roles, and geographies, all while staying compliant and moving fast.
Study protocols are evolving in real time. Participants expect more from the experience. And treatments now require intricate handoffs between hospitals, labs, and manufacturers. Yet many teams still rely on spreadsheets, email, and siloed systems to manage these mission-critical workflows.
The result? Site activation delays, participant drop-off, and costly scheduling errors that can derail both trials and therapy delivery.
Life Sciences Cloud offers a smarter approach. It brings together study management, participant workflows, and advanced therapy orchestration in one real-time platform designed for speed, adaptability, and traceability.
Distinct Roles, Shared Tools
Life Sciences Cloud supports the full spectrum of roles involved in clinical research and therapy delivery. Whether running traditional trials or coordinating cell and gene therapies, each team works from the same unified platform, and their experience is tailored to match their responsibilities and context.
Team |
Responsibility |
|
|---|---|---|
Clinical operations managers |
Oversee study startup, protocol execution, and site performance. |
|
Recruiters and coordinators |
Manage participant outreach, eligibility, and enrollment. |
|
Site teams |
Carry out trial activities on the ground, capturing data and ensuring compliance. |
|
Advanced therapy teams |
Coordinate logistics across collection, manufacturing, and infusion (the process of delivering the therapy back into the patient’s body). |
|
Admins and architects |
Design workflows, configure business logic, and manage integrations with low-code tools. |
|
Along with providing interfaces and workflows for these roles, Life Sciences Cloud supports every phase of clinical trial execution. Here's how it all fits together.
Identify and Activate Study Sites
Launching a study begins with choosing the right sites. This selection involves identifying sites with proven performance, access to eligible participants, and readiness to operate under the protocol. But pinpointing and activating those sites often takes too long. Manual feasibility reviews, misaligned outreach, and scattered activation steps can delay trial startup and increase sponsor burden.
Life Sciences Cloud accelerates this process by combining intelligent site selection with guided activation workflows. The platform helps clinical teams find qualified sites, generate tailored feasibility assessments, and activate startup activities from a shared system.
Agentforce surfaces high-performing sites based on therapeutic area, historical metrics, and target population access. Once potential sites are identified, teams can quickly create questionnaires using generative AI to assess site feasibility and readiness. Starting from a short synopsis or prompt, the system generates a customized set of questions aligned to trial phase, protocol complexity, and regional context.
This example shows a form for US partner sites, with questions grouped under sections like Site Experience, Patient Population, and Infrastructure. Each section can be expanded to capture detailed responses, making it easier to evaluate whether a site is a good fit for a clinical trial.
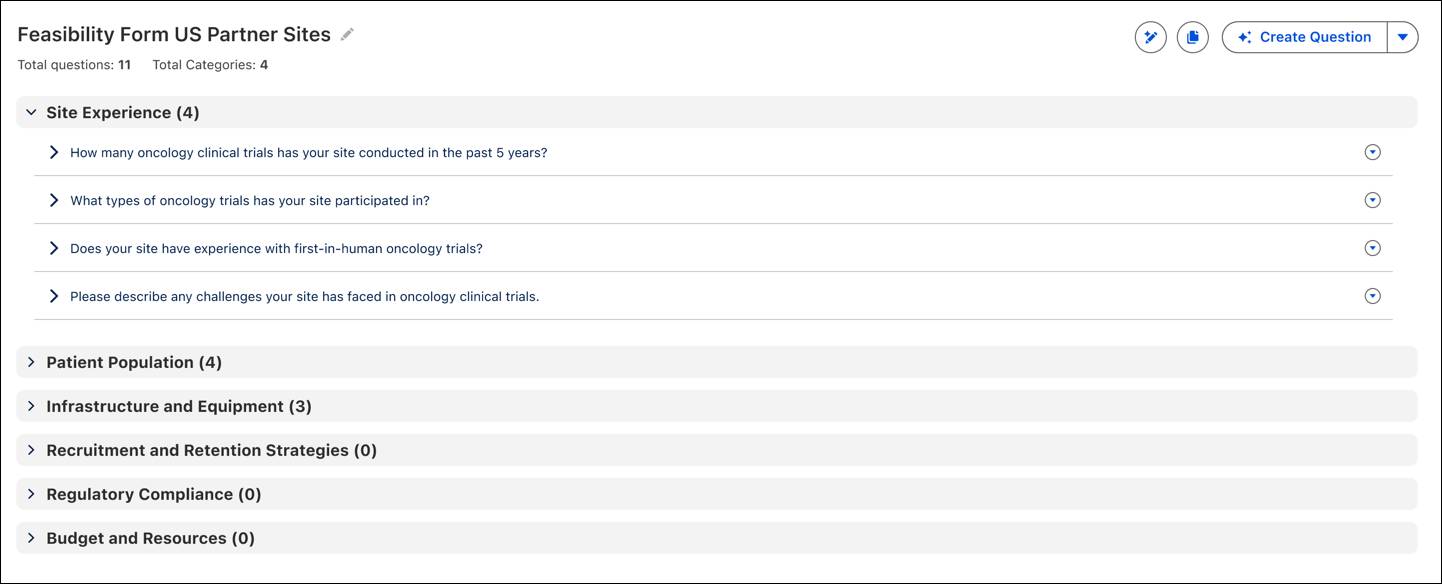
Each output includes structured response types, scoring logic, and support for multiple languages, saving time while providing consistent analysis across global sites.
At Cumulus Pharma, the clinical operations team uses the site-selection feature to support a Phase 3 respiratory study across North America and Europe. Within minutes, they create a region-specific questionnaire that covers enrollment targets, staffing capacity, and regulatory readiness.
After the team identifies and selects the preferred sites, Life Sciences Cloud helps coordinate activation through milestone tracking and shared workflows. Tasks like contract execution, regulatory submissions, and staff training are all managed in one place, keeping sponsors, CROs, and site teams aligned.
Recruit and Enroll Participants
With the trial sites activated, it’s time to bring participants into the study. Life Sciences Cloud supports a seamless end-to-end recruitment and enrollment experience combining public-facing portals, guided prescreening, digital consent, and automated enrollment workflows.
Publish Trials and Empower Participant Discovery
Sponsors can publish active studies through a public-facing Experience Cloud portal, giving potential participants a central place to explore open trials. The portal uses criteria-based search powered by Data Processing Engine to match participants to studies based on demographics, location, and trial attributes like phase, therapeutic area, and inclusion criteria.
Let’s look at how Cumulus Pharma uses this to recruit for its Phase 3 respiratory trial.
A potential participant visits the Cumulus trial portal and filters studies by location and condition. The respiratory study appears along with details on trial phase, eligibility criteria, and participating sites.
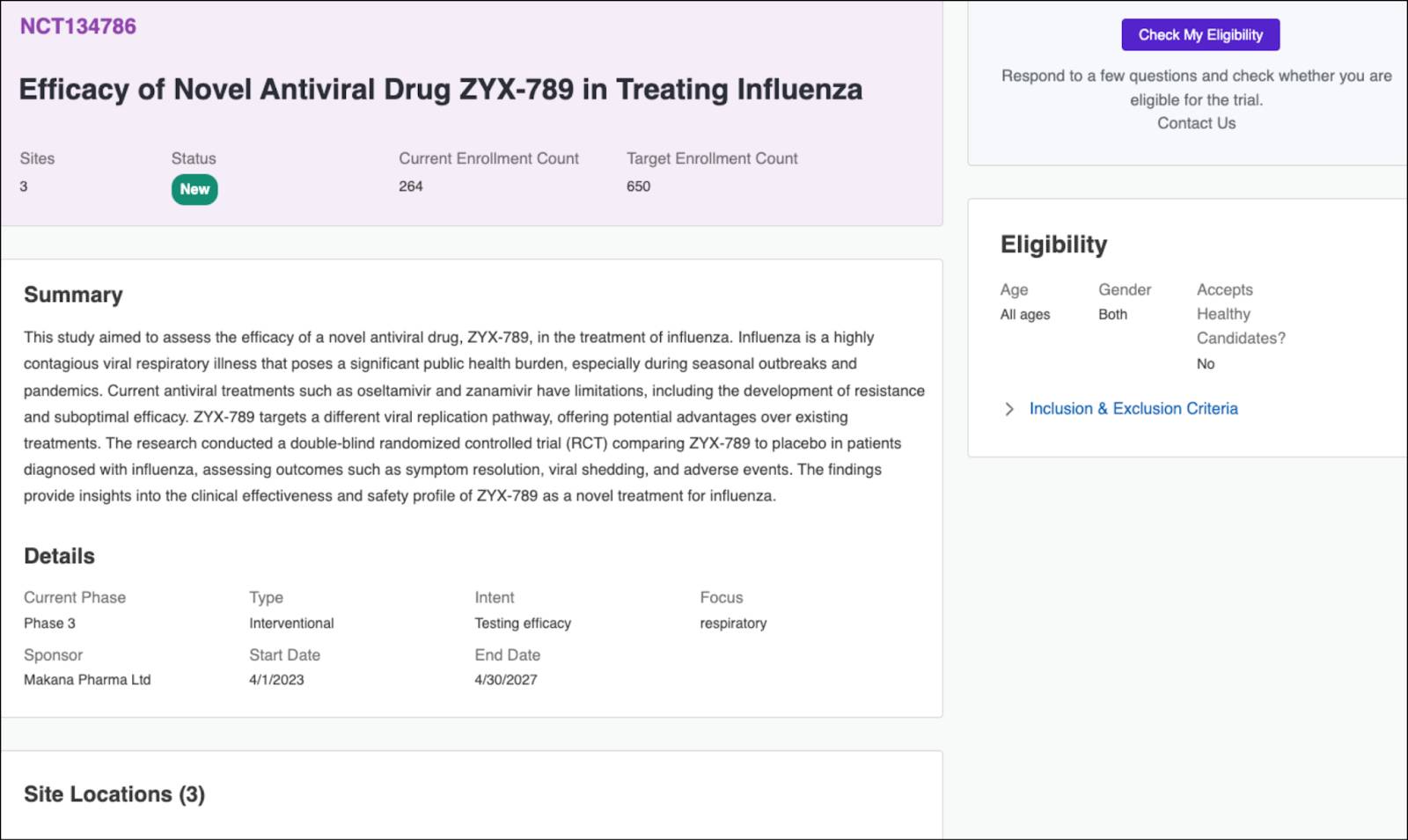
When a participant finds a suitable study, they click Check My Eligibility to launch a guided prescreener.
Guide Prescreening with Smart Flows
Using OmniStudio tools and Discovery Framework, clinical teams build personalized assessment flows that walk candidates through prescreening. These flows collect biographic data, medical history, and lifestyle inputs, and then evaluate candidate eligibility in real time using Business Rules Engine and expression sets.
For example, the participant interested in the influenza study completes the screener and is instantly informed that they qualify.
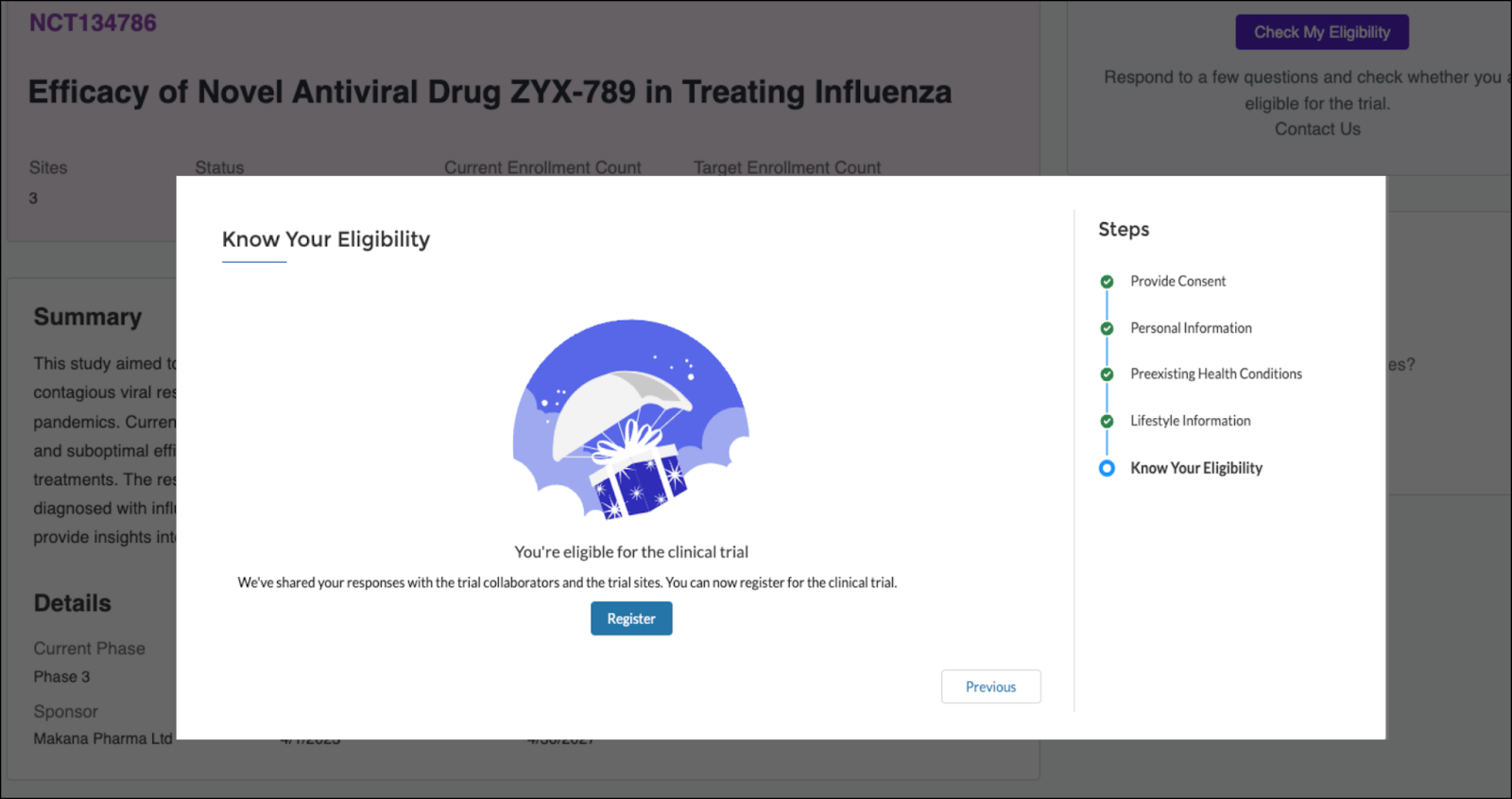
The participant’s information is transformed into structured records so that the site coordinator can begin onboarding.
Digitize Consent and Track Enrollment Milestones
After prescreening, site coordinators use built-in workflows to manage key enrollment steps, such as collecting eConsent, scheduling visits, and advancing participants through defined milestones. Each stage is orchestrated through automated tools that assign tasks and surface status in real time.
In the Cumulus study, the coordinator sends a secure eConsent form to the participant, who signs electronically using a credentialed method that’s compliant with 21 CFR Part 11.
From there, the coordinator schedules a screening visit and tracks progress through structured, auditable enrollment stages.
By standardizing these flows across sites, Cumulus ensures a participant-friendly experience while adhering to regulations and accelerating study timelines.
Coordinate Advanced Therapy Delivery
For personalized treatments like CAR T-cell or autologous gene therapies, delivering care is a logistical high-wire act. Each therapy must move seamlessly from patient collection to manufacturing to reinfusion, on time, under strict conditions, and with no room for error.
Life Sciences Cloud reduces this complexity through three key features designed to help life sciences teams plan, schedule, and monitor each step of personalized therapy delivery.
Define the Patient Journey with Orchestration Frameworks
Therapy workflows start with the Therapy Orchestration Framework. Clinical teams configure the stages of care—like enrollment, apheresis, which is the process of collecting a patient’s blood cells for processing, manufacturing, and administration. Then teams can assign roles and tasks to the right users at the right phase.
In this example, the patient is at the enrollment stage, with tasks such as requesting and confirming consent listed on the left. Across the top, you can see the therapy journey laid out from enrollment through infusion, helping care teams track progress at every stage.
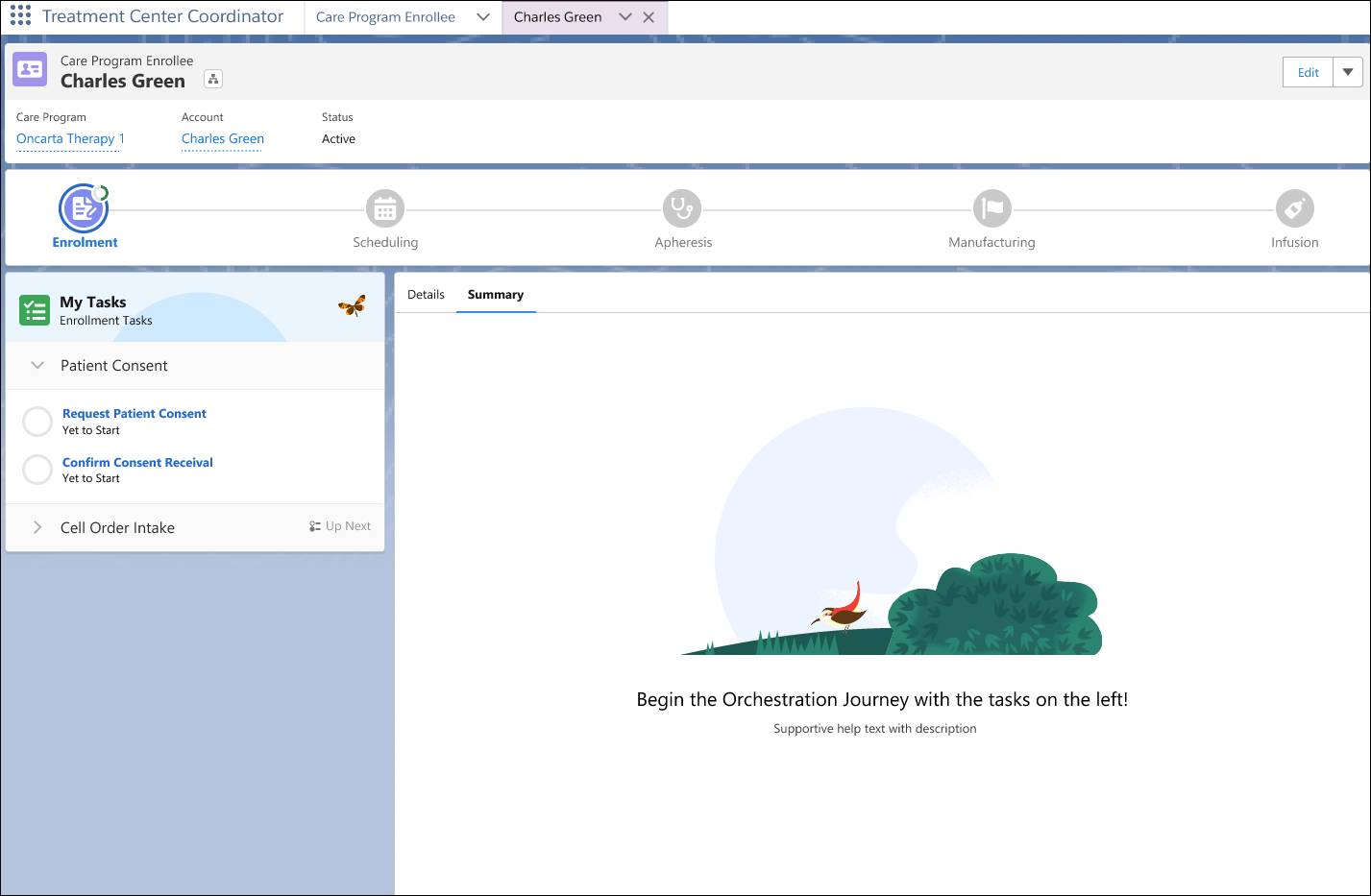
Each team member only sees what they need. For example, an apheresis nurse can view their precollection checklist, while a manufacturing coordinator only has access to their downstream steps.
This role-based visibility helps prevent missed tasks, duplicate data entry, or protocol deviations.
Schedule Complex Therapies with Built-in Guardrails
Advanced therapies involve tightly coupled steps across locations, often with time-sensitive constraints. With Multi-Step Scheduling, Life Sciences Cloud gives coordinators the ability to book the full sequence, from collection, transport, manufacturing, and reinfusion, in a single flow.
This image shows the scheduler, where available time slots for intake, apheresis, manufacturing, and infusion are displayed together.
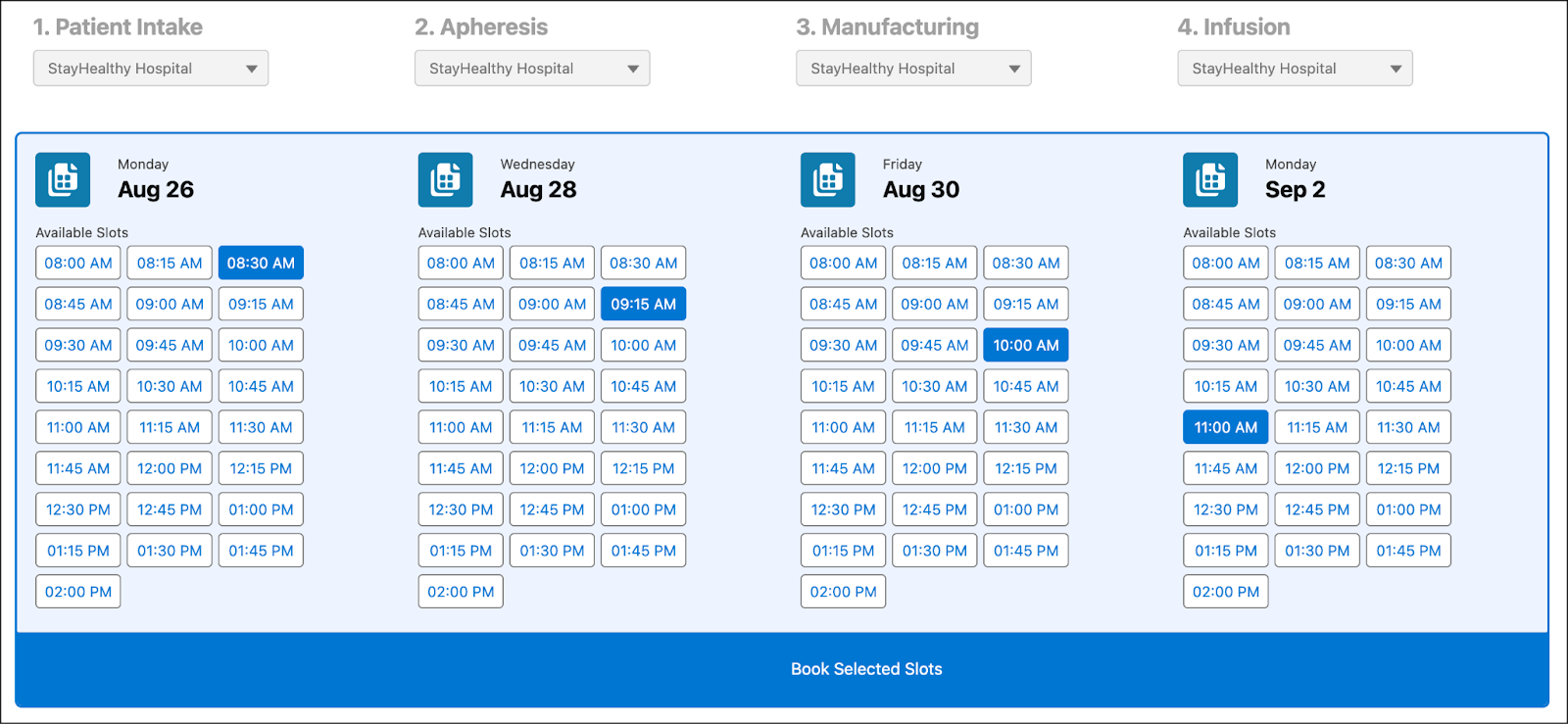
Built-in logic ensures protocol rules are followed. If a patient’s cell collection is scheduled for Monday, the system automatically filters manufacturing slots to begin no earlier than Wednesday, accounting for lead time, transport duration, and capacity limits.
If you or the patient need to reschedule, the platform automatically shifts all dependent appointments forward while keeping the correct sequence. For example, moving the apheresis date also adjusts the manufacturing and infusion slots, so every stage remains aligned with protocol and compliance requirements.
Maintain Chain of Custody and Identity with Confidence
From the first sample draw to final administration, every material transfer must be traceable. Life Sciences Cloud enforces this with built-in chain-of-custody and chain-of-identity tracking. These tools log every handoff, document every action, and ensure that each therapy is matched to the correct patient.
Organizations can define digital verification requirements—like dual sign-off for cell collection—before advancing to the next stage.
This example shows a Digital Verification Setup record for apheresis collection. The configuration specifies a dual-signature requirement, set to run in parallel and tied to the patient’s work order. These details define the checkpoint rules before the next step can begin.
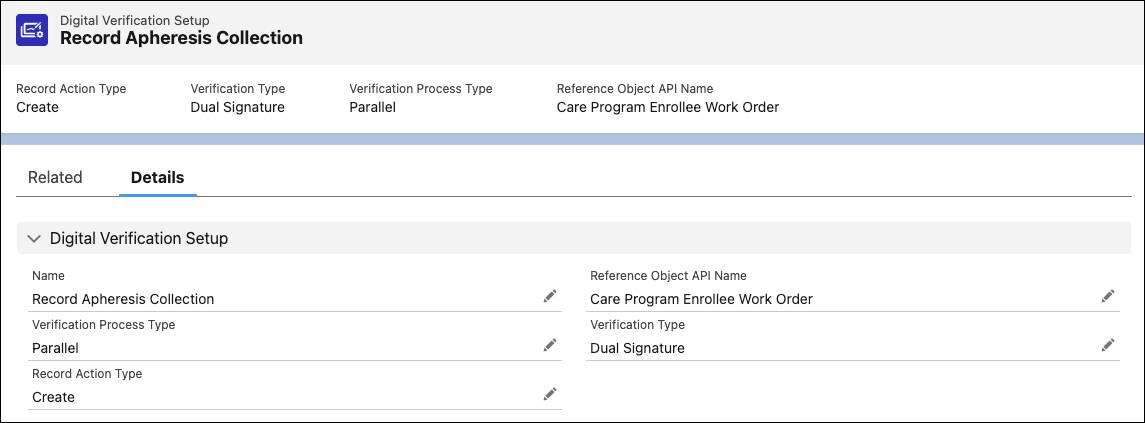
These checkpoints help ensure compliance, improve audit readiness, and build trust across clinical, logistics, and manufacturing teams.
Scale from Trial to Treatment on a Unified Platform
While these capabilities are vital for running complex trials, they’re just as critical in commercial settings. Many organizations use the same sites and partners across both phases.
Life Sciences Cloud supports this reality by unifying clinical and commercial delivery in one system. Reusable templates, region-specific logic, and API-based integrations with third-party logistics and manufacturing systems make it easy to adapt as programs grow.
With this model, teams can shorten site onboarding times, reduce technology sprawl, and maintain therapy continuity from trial to treatment.
A Smarter Approach to Clinical Operations
Operational excellence is what turns complex trials and therapies into real-world success. Life Sciences Cloud gives clinical teams the structure, adaptability, and coordination they need, whether selecting sites, enrolling participants, or orchestrating advanced therapies with strict chain-of-custody controls. By uniting these workflows in one platform, organizations can move faster, minimize errors, and keep studies and treatments on track from first activation to final delivery.
In the next unit, find out how Life Sciences Cloud supports end-to-end patient services that help individuals access therapy, coordinate with providers, and manage the finances and logistics that keep care on track. Follow Cumulus as the healthcare provider launches support services for its newly approved respiratory treatment.